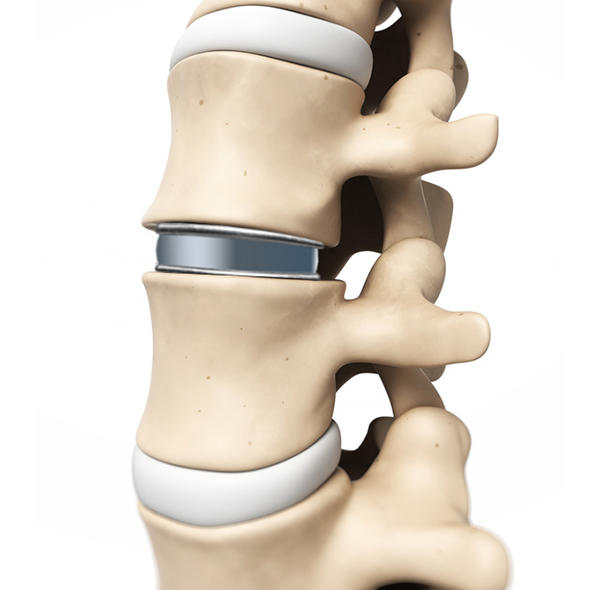
Artificial lumbar disc prosthesis
Intervertebral disc endoprostheses – a movement and function-preserving surgical method for the spine
The implantation of a disc prosthesis (artificial replacement) is a globally accepted method for the treatment of symptomatic degenerative disease of the intervertebral discs. A minimum of 6 months of conservative therapy should be tried beforehand. If this fails, or if any uncontrollable pain or nerve disorders arise, health insurance firms in Switzerland cover the costs of the operation providing that no more than two discs are affected and there is no primary osteoarthritis of the facet joints.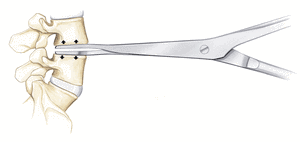 The operation is performed under a general anesthetic administered through the abdominal wall. Depending on the nature and height of the diseased intervertebral disc, the approach to the spine is either through a lower abdominal cross-sectional incision or a longitudinal incision in the skin of about 4-6 cm. As a rule, the peritoneum remains intact, so that disruptions to the intestines can be avoided. Once the large vessels and nerves – the abdominal aorta and vena cava large – located to the front of the spine have been carefully pushed aside, the affected intervertebral disc is exposed. The anterior longitudinal ligament and the intervertebral disc ring can be opened and the defective disc removed completely.
The operation is performed under a general anesthetic administered through the abdominal wall. Depending on the nature and height of the diseased intervertebral disc, the approach to the spine is either through a lower abdominal cross-sectional incision or a longitudinal incision in the skin of about 4-6 cm. As a rule, the peritoneum remains intact, so that disruptions to the intestines can be avoided. Once the large vessels and nerves – the abdominal aorta and vena cava large – located to the front of the spine have been carefully pushed aside, the affected intervertebral disc is exposed. The anterior longitudinal ligament and the intervertebral disc ring can be opened and the defective disc removed completely. 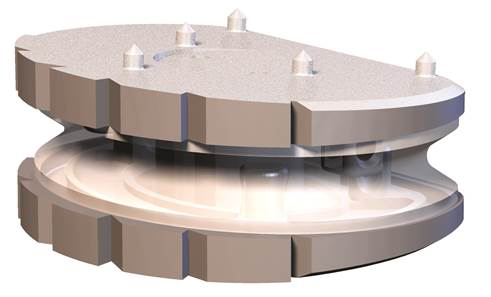 The viscoelastic disc prosthesis (VTDR) Freedom ™ Lumbar Disc (FLD) for the lumbar spine or Freedom Cervical Disc (FCD) for the cervical spine is a novel viscoelastic disc prosthesis to replace a worn disc. The unique design of the latest generation of this prosthesis has the following advantages: – restoration of normal mobility and strength of the affected segment of movement. – Realistic 3D imitation of the human intervertebral disc. – Normal shock absorbing effect like that of the natural intervertebral disc. – Full load capacity on the spine. – Restoring of the natural function of spinal joints, muscles, ligaments and tendons.
The viscoelastic disc prosthesis (VTDR) Freedom ™ Lumbar Disc (FLD) for the lumbar spine or Freedom Cervical Disc (FCD) for the cervical spine is a novel viscoelastic disc prosthesis to replace a worn disc. The unique design of the latest generation of this prosthesis has the following advantages: – restoration of normal mobility and strength of the affected segment of movement. – Realistic 3D imitation of the human intervertebral disc. – Normal shock absorbing effect like that of the natural intervertebral disc. – Full load capacity on the spine. – Restoring of the natural function of spinal joints, muscles, ligaments and tendons.
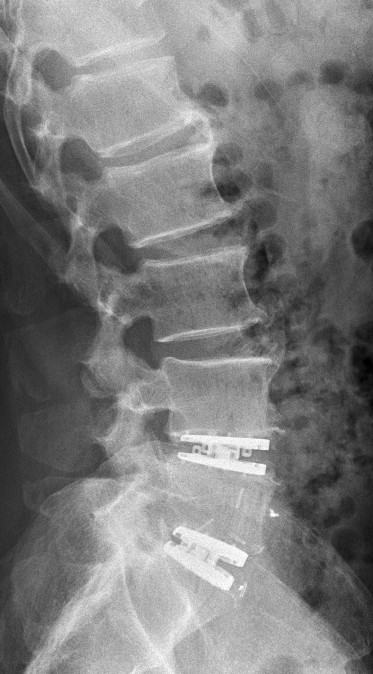
Implantation of two visco-elastic intervertebral disc prostheses 4 to 5 lumbar vertebrae, and between the fifth lumbar vertebra and the sacrum.
What happens after surgery?
After surgery you will stay in hospital for a few days . In general, these are about 3-4 days after an operation on the cervical spine and about 6-7 days after an operation on the lumbar spine. Because the prosthesis is usually instantly motion- and pressure-resistant, you can get up one day after the procedure. In contrast to fusion surgery you are able to sit at this point, but should avoid bending forward under loads, overstretching, lifting heavy objects and abrupt twisting of the spine. However, “natural” movements are allowed. Special treatment (physiotherapy, mobilization, muscle building etc) then follows the operation. As a rule, the prosthesis has grown into the bone after about 3 months. This is checked by x-ray. Obligatory follow-up checks are carried out regularly and the findings are documented in the SWISS spine register, which guarantees the maximum safety and after-care in this operation method.
Literature
Berg. S., Tullberg, T., Branth, B., et al (2009) Total Disc Replacement Compared to Lumbar Fusion: a Randomised Controlled Trial With 2-Year Follow-Up, European Spine Journal, Volume 18, Issue 10, Seite 1512–1519, Springer-Verlag Bertagnoli R; Yue J; McAfee, P.C., An, H.S.; (2011) Bewegungserhaltende Wirbelsäulenchirurgie, Urban&Fischer, München Bertagnoli R; Yue J; Nanieva R; Fenk-Mayer A; Husted DS; Shah RV and Emerson JW.(2006) Lumbar Total Disc Arthroplasty in Patients Older Than 60 Years of Age: a Prospective Study of the ProDisc Prosthesis with 2-Year Minimum Follow-Up Period. J Neurosurg: Spine; 4: 85-90 Bertagnoli R; Yue J; Fenk-Mayer A; Eerulkar J and Emerson JW. (2006)Treatment of Symptomatic Adjacent-Segment Degeneration after Lumbar Fusion with Total Disc Arthroplasty by Using the ProDisc Prosthesis: a Prospective Study with 2-Year Minimum Follow-Up. J Neurosurg: Spine ; 4:91-7 Bertagnoli R., et al. (2005) The Treatment of Disabling Single-Level Lumbar Discogenic Low Back Pain With Total Disc Arthroplasty Utilizing the Prodisc Prosthesis, SPINE, Volume 30, Issue19, Page 2230-2236, Lippincott Williams & Wilkins Inc., Philadelphia Blumenthal S, McAfee PC, Guyer RD et al (2005) A prospective, Randomized, Multicenter Food and Drug Administration Investigational Device Exemptions Study of Lumbar Total Disc Replacement with the CHARITE Artificial Disc Versus Lumbar Fusion: part I: Evaluation of Clinical Outcomes, Spine Volume 30, Number 14, Seite 1565–1575, Lippincott Williams & Wilkins, Inc. Büttner-Janz, K., Schellnack, K., Zippel, H. (1989) Biomechanics of the SB Charité Lumbar Intervertebral Disc Endoprosthesis, International Orthopaedics, Volume 13, Issue 3, Page 173 – 176, Springer Verlag Guyer, R. D., McAfee, P. C., Banco, R. J., et al. (2009) Prospective, Randomized, Multicenter Food and Drug Administration Investigational Device Exemption Study of Lumbar Total Disc Replacement with the CHARITÉ Artificial Disc Versus Lumbar Fusion: Five-year Follow-Up, Spine-Journal, Volume 9, Page 374 – 386 Mayer, H.M (2005), Total Lumbar Disc Replacement , Journal of Bone and Joint Surgery, 87 Schluessmann, E., et al (2009) SWISSspine: a Nationwide Registry for Health Technology Assessment of Lumbar Disc Prostheses, European Spine Journal, Volume 18, Issue 6, Page 851 – 861, Springer Link Verlag, Berlin Heidelberg New York Serhan, H., et al. (2011) Lumbar Arthroplasty, Motion-Preserving Technologies for Degenerative Lumbar Spine: The Past, Present, and Future Horizons, SAS Journal, Volume 5, Issue 3, Page 75 – 89, Elsevier Inc. Zigler, J., Delamarter, R., Spivak, J. M., et al. (2007) Results of the Prospective, Randomized, Multicenter Food and Drug Administration Investigational Device Exemption Study of the ProDisc